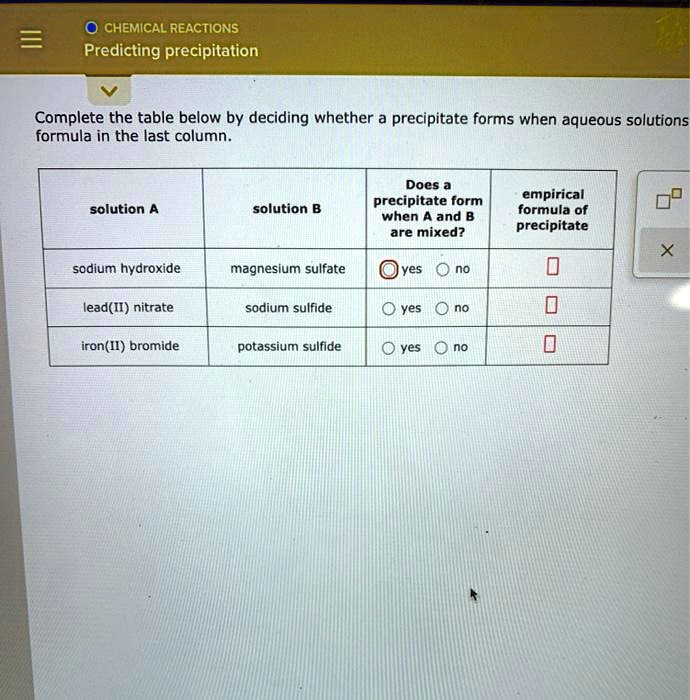
Magnesium Bromide And Potassium Hydroxide Precipitate . in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. the first step in the preparation of magnesium metal is the precipitation of mg(oh) 2 from sea water by the addition. mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where one mole of aqueous magnesium bromide.
from www.numerade.com
in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. the first step in the preparation of magnesium metal is the precipitation of mg(oh) 2 from sea water by the addition. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where one mole of aqueous magnesium bromide. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed.
SOLVED CHEMICAL REACTIONS Predicting precipitation Complete the table
Magnesium Bromide And Potassium Hydroxide Precipitate the first step in the preparation of magnesium metal is the precipitation of mg(oh) 2 from sea water by the addition. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. the first step in the preparation of magnesium metal is the precipitation of mg(oh) 2 from sea water by the addition. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where one mole of aqueous magnesium bromide. complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed.
From www.sigmaaldrich.co.th
THIAZOLYL BLUE TETRAZOLIU M BROMIDE, 98 Merck Life Sciences Thailand Magnesium Bromide And Potassium Hydroxide Precipitate a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. the first step in the preparation of magnesium metal is the precipitation of mg(oh) 2 from sea water by the addition. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous.. Magnesium Bromide And Potassium Hydroxide Precipitate.
From www.chemkits.eu
Potassium bromide, 98.5, 7758023 Magnesium Bromide And Potassium Hydroxide Precipitate mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where one mole of aqueous magnesium bromide. the first step in the preparation of magnesium metal is the precipitation of mg(oh) 2 from sea water by the addition. watch this video to learn about precipitation reactions, a type of chemical reaction that produces. Magnesium Bromide And Potassium Hydroxide Precipitate.
From www.sciencephoto.com
Magnesium Hydroxide Precipitate Stock Image C027/9874 Science Magnesium Bromide And Potassium Hydroxide Precipitate complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid. Magnesium Bromide And Potassium Hydroxide Precipitate.
From sielc.com
Ethidium bromide SIELC Technologies Magnesium Bromide And Potassium Hydroxide Precipitate watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where. Magnesium Bromide And Potassium Hydroxide Precipitate.
From nsilabsolutions.com
Bromide CRM IS019 NSI Lab Solutions Magnesium Bromide And Potassium Hydroxide Precipitate a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where one mole of aqueous magnesium bromide. watch. Magnesium Bromide And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED Texts CHEMICAL REACTIONS Predicting precipitation Complete Magnesium Bromide And Potassium Hydroxide Precipitate in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. the first step in the preparation of magnesium metal is the precipitation of mg(oh) 2 from sea. Magnesium Bromide And Potassium Hydroxide Precipitate.
From www.alamy.com
3D image of Benzododecinium bromide skeletal formula molecular Magnesium Bromide And Potassium Hydroxide Precipitate complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where one mole of aqueous. Magnesium Bromide And Potassium Hydroxide Precipitate.
From byjus.com
Write the molecular formulae for the compound Copper II Bromide Magnesium Bromide And Potassium Hydroxide Precipitate a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. the first step in the preparation of magnesium metal is the precipitation of mg(oh) 2 from sea water by the addition. mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where one mole of aqueous magnesium. Magnesium Bromide And Potassium Hydroxide Precipitate.
From www.sciencecompany.com
Reagent grade Potassium Bromide, 500g for sale. Buy from The Science Magnesium Bromide And Potassium Hydroxide Precipitate complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. . Magnesium Bromide And Potassium Hydroxide Precipitate.
From www.thistlescientific.co.uk
Ethidium Bromide solution 10mg/ml Thistle Scientific Magnesium Bromide And Potassium Hydroxide Precipitate the first step in the preparation of magnesium metal is the precipitation of mg(oh) 2 from sea water by the addition. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when. Magnesium Bromide And Potassium Hydroxide Precipitate.
From testbook.com
Sodium Bromide Learn Definition, Properties, Structure & Uses Magnesium Bromide And Potassium Hydroxide Precipitate the first step in the preparation of magnesium metal is the precipitation of mg(oh) 2 from sea water by the addition. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where one mole of aqueous magnesium. Magnesium Bromide And Potassium Hydroxide Precipitate.
From www.dreamstime.com
Potassium Bromide Chemical Formula on Waterdrop Background Stock Magnesium Bromide And Potassium Hydroxide Precipitate a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two. Magnesium Bromide And Potassium Hydroxide Precipitate.
From www.sciencephoto.com
Magnesium Hydroxide Precipitate Stock Image C027/9873 Science Magnesium Bromide And Potassium Hydroxide Precipitate watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two. Magnesium Bromide And Potassium Hydroxide Precipitate.
From www.indiamart.com
BioTech Grade Powder Sodium Bromide for Petroleum Industry, for Magnesium Bromide And Potassium Hydroxide Precipitate complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where one mole of aqueous magnesium bromide. in. Magnesium Bromide And Potassium Hydroxide Precipitate.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Magnesium Bromide And Potassium Hydroxide Precipitate a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. the first step in the preparation of magnesium metal is the precipitation of mg(oh) 2 from sea water by. Magnesium Bromide And Potassium Hydroxide Precipitate.
From www.solcohealthcare.com
Rocuronium Bromide Injection Solco Healthcare Magnesium Bromide And Potassium Hydroxide Precipitate the first step in the preparation of magnesium metal is the precipitation of mg(oh) 2 from sea water by the addition. complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where one mole of. Magnesium Bromide And Potassium Hydroxide Precipitate.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Magnesium Bromide And Potassium Hydroxide Precipitate watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where one mole of aqueous magnesium bromide. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed.. Magnesium Bromide And Potassium Hydroxide Precipitate.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Magnesium Bromide And Potassium Hydroxide Precipitate in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. mgbr2 + koh = kbr + mg (oh)2 is a double displacement (metathesis) reaction where one mole. Magnesium Bromide And Potassium Hydroxide Precipitate.